
Use the information below to generate a citation. Then you must include on every digital page view the following attribution: If you are redistributing all or part of this book in a digital format, Then you must include on every physical page the following attribution: If you are redistributing all or part of this book in a print format, Want to cite, share, or modify this book? This book is This mathematical approach is outlined in this flowchart:įor proper unit cancellation, the 0.500-L volume is converted into 500 mL, and the mass percentage is expressed as a ratio, 37.2 g HCl/g solution: Using the solution density given, convert the solution’s volume to mass, and then use the given mass percentage to calculate the solute mass. In order to derive the mass of solute in a solution from its mass percentage, the mass of the solution must be known. Since the solution density isn’t greatly different from that of water (1 g/mL), a reasonable estimate of the HCl mass in 500 g (0.5 L) of the solution is about five times greater than that in a 100 g portion, or 5 × × 40 = 200 g. The HCl concentration is near 40%, so a 100-g portion of this solution would contain about 40 g of HCl. What mass of HCl is contained in 0.500 L of this solution? The density of this solution is 1.19 g/mL. “Concentrated” hydrochloric acid is an aqueous solution of 37.2% HCl that is commonly used as a laboratory reagent.

The mass percentage of a solution component is defined as the ratio of the component’s mass to the solution’s mass, expressed as a percentage: Percentages are also commonly used to express the composition of mixtures, including solutions. Mass PercentageĮarlier in this chapter, percent composition was introduced as a measure of the relative amount of a given element in a compound.

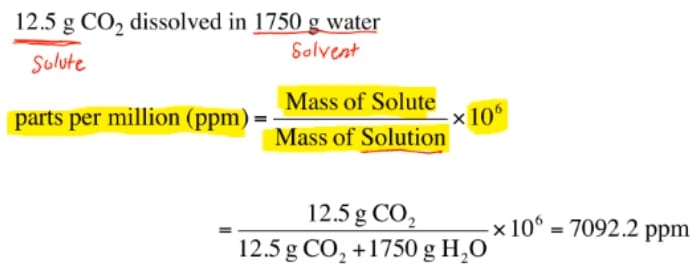
This section will describe some other units of concentration that are commonly used in various applications, either for convenience or by convention. However, molarity is only one measure of concentration. The previous section introduced molarity, a very useful measurement unit for evaluating the concentration of solutions. Perform computations relating a solution’s concentration and its components’ volumes and/or masses using these units.Define the concentration units of mass percentage, volume percentage, mass-volume percentage, parts-per-million (ppm), and parts-per-billion (ppb).Thus for the 1 M NaCl, the total ion concentration is 2 M for the 1 M CaCl 2, the total ion concentration is 3 M.By the end of this section, you will be able to: In addition, the total ion concentration is the sum of the individual ion concentrations. However, if the solution were 1 M CaCl 2, there are two Cl −(aq) ions for every formula unit dissolved, so the concentration of Cl −(aq) would be 2 M, not 1 M. For example, if 1 M NaCl were prepared, the solution could also be described as a solution of 1 M Na +(aq) and 1 M Cl −(aq) because there is one Na + ion and one Cl − ion per formula unit of the salt.

Because the ions in ionic compounds go their own way when a compound is dissolved in a solution, the resulting concentration of the ion may be different from the concentration of the complete salt. What mass of Fe 3+ ion is present in 3,450 mL of H 2O, which has a density of 1.00 g/mL?įor ionic solutions, we need to differentiate between the concentration of the salt versus the concentration of each individual ion. The concentration of Fe 3+ ion in a sample of H 2O is 335.0 ppm.


 0 kommentar(er)
0 kommentar(er)
